Disease Overview
-
Think Fabry, think GL-3 accumulation
Pathogenic variants in the GLA gene lead to deficiency of α-GAL A activity resulting in GL-3 and lyso-GL3 accumulation in various tissues.1,2 Accumulation of GL-3 in vascular endothelia causes tissue damage in a number of organ systems and is regarded as a distinguishing feature of Fabry disease.3
Accumulation of GL-3 and increased burden in classic Fabry disease over time4

α-GAL A, α-galactosidase A; GLA, galactosidase alpha; GL-3, globotriaosylceramide.
Think Fabry, think GL-3 accumulation
Pathogenic variants in the GLA gene lead to deficiency of α-GAL A activity resulting in GL-3 and lyso-GL3 accumulation in various tissues.1,2 Accumulation of GL-3 in vascular endothelia causes tissue damage in a number of organ systems and is regarded as a distinguishing feature of Fabry disease.3
Accumulation of GL-3 and increased burden in classic Fabry disease over time4

α-GAL A, α-galactosidase A; GLA, galactosidase alpha; GL-3, globotriaosylceramide.
-
Think Fabry, think renal involvement that may present early in life and could go undetected
Renal manifestations
- Early and progressive GL-3 accumulation can lead to irreversible organ damage and potentially life-threatening clinical events5
- Renal damage may occur with podocyte injury, starting as early as the first decade of life, despite minimal or normal microalbuminuria6,7
- Renal biopsy can provide direct assessment of the level of pathological structural changes and may help guide the decision of when to initiate treatment8
Adapted from Schiffmann R et al, 2009; Ortiz A et al, 2008; Germain DP, 2010; Ramaswami U et al, 2010; Eng CM et al, 2006; Tøndel C et al, 2013; and Banikazemi M et al, 2007.1,5,7,9-12
*Accumulation of GL-3 starts in utero.6
ESRD, end-stage renal disease; GL-3, globotriaosylceramide.See how Fabry could be linked to unexplained chronic kidney disease
Think Fabry, think renal involvement that may present early in life and could go undetected
Renal manifestations
- Early and progressive GL-3 accumulation can lead to irreversible organ damage and potentially life-threatening clinical events5
- Renal damage may occur with podocyte injury, starting as early as the first decade of life, despite minimal or normal microalbuminuria6,7
- Renal biopsy can provide direct assessment of the level of pathological structural changes and may help guide the decision of when to initiate treatment8
Adapted from Schiffmann R et al, 2009; Ortiz A et al, 2008; Germain DP, 2010; Ramaswami U et al, 2010; Eng CM et al, 2006; Tøndel C et al, 2013; and Banikazemi M et al, 2007.1,5,7,9-12
*Accumulation of GL-3 starts in utero.6
ESRD, end-stage renal disease; GL-3, globotriaosylceramide.See how Fabry could be linked to unexplained chronic kidney disease
-
Think Fabry, think cardiac symptoms before serious complications occur13-15
Cardiac manifestations over time
EKG abnormalities in Fabry disease include:1,16,17
- Bradycardia, which is a common finding in adults and frequently present in children
- Voltage criteria and repolarization changes related to left ventricular hypertrophy and/or remodeling
- ST segment depression
- T-wave inversions
- Shortened P-wave duration, which is one of the earliest signs of cardiac involvement15
- Enlarged QRS complex and prolonged QTc intervals, which have been associated with advancing Fabry disease15
Adapted from Schiffmann R et al, 2009; Ortiz A et al, 2008; Germain DP, 2010; Ramaswami U et al, 2010; Eng CM et al, 2006; Tøndel C et al, 2013; and Banikazemi M et al, 2007.1,5,7,9-12
*Accumulation of GL-3 starts in utero.6
ESRD, end-stage renal disease; GL-3, globotriaosylceramide.See how Fabry could be linked to unexplained hypertrophic cardiomyopathy
Think Fabry, think cardiac symptoms before serious complications occur13-15
Cardiac manifestations over time
EKG abnormalities in Fabry disease include:1,16,17
- Bradycardia, which is a common finding in adults and frequently present in children
- Voltage criteria and repolarization changes related to left ventricular hypertrophy and/or remodeling
- ST segment depression
- T-wave inversions
- Shortened P-wave duration, which is one of the earliest signs of cardiac involvement15
- Enlarged QRS complex and prolonged QTc intervals, which have been associated with advancing Fabry disease15
Adapted from Schiffmann R et al, 2009; Ortiz A et al, 2008; Germain DP, 2010; Ramaswami U et al, 2010; Eng CM et al, 2006; Tøndel C et al, 2013; and Banikazemi M et al, 2007.1,5,7,9-12
*Accumulation of GL-3 starts in utero.6
ESRD, end-stage renal disease; GL-3, globotriaosylceramide.See how Fabry could be linked to unexplained hypertrophic cardiomyopathy
-
Think Fabry, think neurologic symptoms before serious consequences result
Neurologic manifestations over time1,18-20
Differentiating Fabry disease from multiple sclerosis
- As many as 7% of people with Fabry disease were previously misdiagnosed with multiple sclerosis due to overlapping presentation21-23
- Absence of infratentorial and corpus collosum lesions on MRI can aid in the differential diagnosis between MS and Fabry disease24
GI=gastrointestinal; CNS=central nervous system; MS=multiple sclerosis.
See significantly increased stroke risk for males and females with Fabry vs general population
Think Fabry, think neurologic symptoms before serious consequences result
Neurologic manifestations over time1,18-20
Differentiating Fabry disease from multiple sclerosis
- As many as 7% of people with Fabry disease were previously misdiagnosed with multiple sclerosis due to overlapping presentation21-23
- Absence of infratentorial and corpus collosum lesions on MRI can aid in the differential diagnosis between MS and Fabry disease24
GI=gastrointestinal; CNS=central nervous system; MS=multiple sclerosis.
See significantly increased stroke risk for males and females with Fabry vs general population
-
Fabry disease results in the accumulation of glycosphingolipids, GL-3 and its deacylated form (without fatty acid), lyso-GL-3.25
The following are key recommendations for the practical application of GL-3 and lyso-GL-3 in clinical practice25
DBS=dried blood spot; ERT=enzyme replacement therapy. *Limited evidence of benefit following change in ERT type in terms of predicting clinical events. Adapted from Burlina A et al. 2023.25
Fabry disease results in the accumulation of glycosphingolipids, GL-3 and its deacylated form (without fatty acid), lyso-GL-3.25
The following are key recommendations for the practical application of GL-3 and lyso-GL-3 in clinical practice25
DBS=dried blood spot; ERT=enzyme replacement therapy. *Limited evidence of benefit following change in ERT type in terms of predicting clinical events. Adapted from Burlina A et al. 2023.25
Think Fabry, think life-threatening, multisystemic disease progression1,2,28
Irreversible damage to multiple vital organs can cause renal, cardiovascular, and cerebrovascular complications if Fabry disease is left untreated.1,2,28
Multisystemic signs and symptoms
- Neuropathic pain
- Pain crises
- Heat and/or cold intolerance
- Hypohidrosis/hyperhidrosis
- Hearing loss/tinnitus
- Dizziness
- Burning of hands and feet
- Angiokeratomas
- Nausea/vomiting
- Diarrhea and constipation
- Abdominal pain and/or bloating
- Difficulty gaining weight in childhood
- Cornea verticillate
- Tortuous vessels (conjunctival)
- Fabry cataract
- Corneal whorling
- Aortic stiffness

- Depression/anxiety
- Fatigue
- Dyspnea
- Wheezing
- Chronic cough
- Shortness of breath
- Progressive LVH
- Chest pain
- Bradycardia
- Cardiomyopathy
- Arrhythmias, some of which can be lethal
- Ventricular fibrosis
- Heart failure
- Pathological albuminuria/proteinuria
- Decreased glomerular filtration rate
- Kidney failure
- Transient ischemic attack
- Early stroke
Patients with Fabry disease experience an approximated 16-year reduction in lifespan for males and a 5- to 14-year reduction for females compared with the general population36-38
Think Fabry during diagnostic workups
See how Fabry may present in your practice
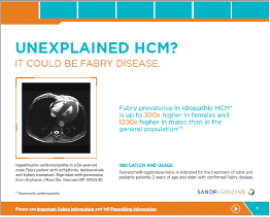
Cardiology Brochure
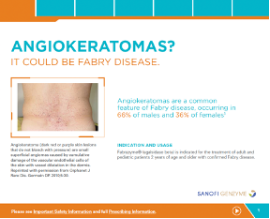
Dermatology Brochure

Nephrology Brochure

Neurology Brochure
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
|
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy. Initiate FABRAZYME in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g. anaphylaxis) occurs, discontinue FABRAZYME and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur [see Warnings and Precautions (5.1)]. |
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions Including Anaphylaxis
In clinical trials and post-marketing experience, approximately 1% of patients developed anaphylactic or severe hypersensitivity reactions, some life-threatening, during Fabrazyme infusion. Reactions have included localized angioedema (including swelling of the face, mouth, and throat), bronchospasm, hypotension, generalized urticaria, dysphagia, rash, dyspnea, flushing, chest discomfort, pruritus, and nasal congestion. Consider pretreating with antihistamines, antipyretics, and/or corticosteroids; however, reactions may still occur.
In Fabrazyme clinical trials, some patients developed IgE antibodies or skin test reactivity specific to Fabrazyme.
- Higher incidences of hypersensitivity reactions were observed in adult patients with persistent anti-Fabrazyme antibodies, and in those with high antibody titers compared with antibody negative adult patients.
- Consider testing for IgE antibodies in patients who experienced suspected hypersensitivity reactions and consider the risks and benefits of continued treatment in patients with anti-Fabrazyme IgE antibodies. Rechallenge of these patients should only occur under the direct supervision of qualified personnel, with appropriate medical support measures readily available.
Infusion-Associated Reactions
In Fabrazyme clinical trials, 59% of patients experienced infusion-associated reactions (IARs), some of which were severe. IARs are defined as those occurring on the same day as the infusion. The incidence of these reactions was higher in patients who were positive for anti-Fabrazyme antibodies than those negative for anti-Fabrazyme antibodies.
- Consider pretreatment with antipyretics, antihistamines, and/or corticosteroids to reduce the risk of IARs; however, they may still occur.
- If a mild or moderate IAR occurs, consider holding the infusion temporarily, decreasing the infusion rate, and/or reducing the Fabrazyme dosage. If a severe IAR occurs, discontinue Fabrazyme immediately and initiate appropriate medical treatment as needed. Assess the risks and benefits of readministering Fabrazyme and monitor patients closely if readministering.
- Patients with advanced Fabry disease may have compromised cardiac function, which may predispose them to a higher risk of severe complications from IARs. Closely monitor patients with compromised cardiac function receiving Fabrazyme.
Common Adverse Reactions
Adverse reactions reported (≥20%) were upper respiratory tract infection, chills, pyrexia, headache, cough, paresthesia, fatigue, peripheral edema, dizziness, and rash.
Please see full Prescribing Information, including Boxed WARNING
IMPORTANT SAFETY INFORMATION
Show moreIMPORTANT SAFETY INFORMATION
|
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy. Initiate FABRAZYME in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g. anaphylaxis) occurs, discontinue FABRAZYME and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur [see Warnings and Precautions (5.1)]. |
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions Including Anaphylaxis
In clinical trials and post-marketing experience, approximately 1% of patients developed anaphylactic or severe hypersensitivity reactions, some life-threatening, during Fabrazyme infusion. Reactions have included localized angioedema (including swelling of the face, mouth, and throat), bronchospasm, hypotension, generalized urticaria, dysphagia, rash, dyspnea, flushing, chest discomfort, pruritus, and nasal congestion. Consider pretreating with antihistamines, antipyretics, and/or corticosteroids; however, reactions may still occur.
In Fabrazyme clinical trials, some patients developed IgE antibodies or skin test reactivity specific to Fabrazyme.
- Higher incidences of hypersensitivity reactions were observed in adult patients with persistent anti-Fabrazyme antibodies, and in those with high antibody titers compared with antibody negative adult patients.
- Consider testing for IgE antibodies in patients who experienced suspected hypersensitivity reactions and consider the risks and benefits of continued treatment in patients with anti-Fabrazyme IgE antibodies. Rechallenge of these patients should only occur under the direct supervision of qualified personnel, with appropriate medical support measures readily available.
Infusion-Associated Reactions
In Fabrazyme clinical trials, 59% of patients experienced infusion-associated reactions (IARs), some of which were severe. IARs are defined as those occurring on the same day as the infusion. The incidence of these reactions was higher in patients who were positive for anti-Fabrazyme antibodies than those negative for anti-Fabrazyme antibodies.
- Consider pretreatment with antipyretics, antihistamines, and/or corticosteroids to reduce the risk of IARs; however, they may still occur.
- If a mild or moderate IAR occurs, consider holding the infusion temporarily, decreasing the infusion rate, and/or reducing the Fabrazyme dosage. If a severe IAR occurs, discontinue Fabrazyme immediately and initiate appropriate medical treatment as needed. Assess the risks and benefits of readministering Fabrazyme and monitor patients closely if readministering.
- Patients with advanced Fabry disease may have compromised cardiac function, which may predispose them to a higher risk of severe complications from IARs. Closely monitor patients with compromised cardiac function receiving Fabrazyme.
Common Adverse Reactions
Adverse reactions reported (≥20%) were upper respiratory tract infection, chills, pyrexia, headache, cough, paresthesia, fatigue, peripheral edema, dizziness, and rash.
Please see full Prescribing Information, including Boxed WARNING
INDICATION AND USAGE
Fabrazyme® is indicated for the treatment of adult and pediatric patients 2 years of age and older with confirmed Fabry disease.
References: 1. Germain DP. Orphanet J Rare Dis. 2010;5:30. 2. Ortiz A et al. Mol Genet Metab. 2018;123(4):416-427. 3. Wanner C et al. Mol Genet Metab. 2018;124(3):189-203. 4. Eng CM et al. J Inherit Metab Dis. 2007;30(2):184-192. 5. Eng CM et al. Genet Med. 2006;8(9):539-548. 6. Tøndel C et al. Nephron. 2015;129(1):16-21. 7. Tøndel C et al. J Am Soc Nephrol. 2013;24(1):37-148. 8. Hopkin JR et al. Mol Genet Metab. 2016;117(2):104-113. 9. Schiffmann R et al. Nephrol Dial Transplant. 2009;24(7):2102-2111. 10. Ortiz A et al. Nephrol Dial Transplant. 2008;23(5)1600-1607. 11. Ramaswami U et al. Clin J Am Soc Nephrol. 2010;5(2):365-370. 12. Banikazemi M et al. Ann Intern Med. 2007;146(2):77-86. 13. Seydelmann N et al. Best Pract Res Clin Endocrinol Metab. 2015;29(2):195-204. 14. Chimenti C et al. Hum Pathol. 2015;46(11):1760-1768. 15. Namdar M. Front Cardiovasc Med. 2016;3:1-7. 16. Pieroni M et al. J Am Coll Cardiol. 2021;77(7):923-936. 17. Wilson HC et al. Am J Cardiol. 2017;251-255. 18. Sims K. Stroke. 2009;40:788-794. 19. Fellgiebel A et al. Lancet Neurol. 2006;5(9):791-795. 20. Politei JM et al. CNS Neurosci Ther. 2016;22:568-576. 21. Lidove O et al. Clin Genet. 2012;81(6):571-577. 22. Colomba P et al. Oncotarget. 2018;9(8):7758-7762. 23. Bottcher T et al. PLoS One. 2013;8(8):e71894. 24. Ugga L et al. Brain Behav. 2018;8(11):e01121. 25. Burlina A et al. Mol Genet Metab. 2023;139(2):107585. 26. van der Veen S et al. Clin J Am Soc Nephrol. 2023;18(10):1272-1282. 27. Germain DP et al. Mol Gen Metab. 2022;137:49-61. 28. Wanner C et al. Mol Genet Metab. 2019;126(3):210-211. 29. Lidove O et al. Intl J Clin Pract. 2006;60(9):1053-1059. 30. Burlina A et al. BMC Neurol. 2011;11(61). 31. Sodi A et al. Br J Ophthalmol. 2007;91(2):210-214. 32. Zarate Y, Hopkin R. Lancet. 2008;372:1427-1435. 33. Yousef Z et al. Eur Heart J. 2013;34(11):802-808. 34. Linhart A et al. J Inherit Metab Dis. 2001;24(2):75-83. 35. Shi Q et al. J Stroke Cerebrovasc Dis. 2014;25(5):985-992. 36. Waldek S et al. Genet Med. 2009;11(11):790-796. 37. Mehta A et al. J Med Genet. 2009;46(8):548-552. 38. Arias E et al. NVSS Vital Statistics Rapid Release Report No. 015. 2021;1-12. Available at: https://www.researchgate.net/publication/362689060_Provisional_Life_ Expecta ncy_Estimates_for_2020. Accessed: May 2024.

