Downloadable Content:
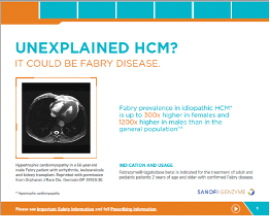
Cardiology Brochure
Important information about cardiac disease, Fabry, and Fabrazyme.

Neurology Brochure
The facts about cerebrovascular complications, Fabry, and Fabrazyme.
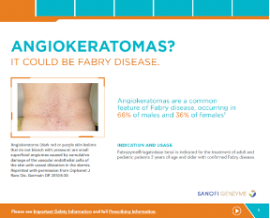
Dermatology Brochure
Guide to dermatological effects, Fabry, and Fabrazyme.

Nephrology Brochure
Guide to renal implications, Fabry, and Fabrazyme.

CareConnect Co-pay Program
Application form to sign up for CareConnect Co-pay Program.

Fabrazyme Billing and Reimbursement Guide
Information about billing and reimbursement regarding Fabrazyme.

Fabrazyme Preparation & Administration Sheet
Information to guide the preparation and administration of Fabrazyme.

Fabrazyme Suggested Infusion Supply List
Suggested supplies for the preparation and administration of Fabrazyme infusions.

Fabrazyme Fact Sheet
Information about Fabrazyme, including product codes.

CareConnect Personalized Support Services HCP Brochure
Information about CareConnect and its offerings.

“It is important to be consistent with therapy. Of course, patients are ultimately responsible for their own health outcomes, so they need to take managing this disease seriously and be as involved as possible.”
Marie, a real Fabrazyme patient for over 20 years

IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
|
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy. Initiate FABRAZYME in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g. anaphylaxis) occurs, discontinue FABRAZYME and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur [see Warnings and Precautions (5.1)]. |
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions Including Anaphylaxis
In clinical trials and post-marketing experience, approximately 1% of patients developed anaphylactic or severe hypersensitivity reactions, some life-threatening, during Fabrazyme infusion. Reactions have included localized angioedema (including swelling of the face, mouth, and throat), bronchospasm, hypotension, generalized urticaria, dysphagia, rash, dyspnea, flushing, chest discomfort, pruritus, and nasal congestion. Consider pretreating with antihistamines, antipyretics, and/or corticosteroids; however, reactions may still occur.
In Fabrazyme clinical trials, some patients developed IgE antibodies or skin test reactivity specific to Fabrazyme.
- Higher incidences of hypersensitivity reactions were observed in adult patients with persistent anti-Fabrazyme antibodies, and in those with high antibody titers compared with antibody negative adult patients.
- Consider testing for IgE antibodies in patients who experienced suspected hypersensitivity reactions and consider the risks and benefits of continued treatment in patients with anti-Fabrazyme IgE antibodies. Rechallenge of these patients should only occur under the direct supervision of qualified personnel, with appropriate medical support measures readily available.
Infusion-Associated Reactions
In Fabrazyme clinical trials, 59% of patients experienced infusion-associated reactions (IARs), some of which were severe. IARs are defined as those occurring on the same day as the infusion. The incidence of these reactions was higher in patients who were positive for anti-Fabrazyme antibodies than those negative for anti-Fabrazyme antibodies.
- Consider pretreatment with antipyretics, antihistamines, and/or corticosteroids to reduce the risk of IARs; however, they may still occur.
- If a mild or moderate IAR occurs, consider holding the infusion temporarily, decreasing the infusion rate, and/or reducing the Fabrazyme dosage. If a severe IAR occurs, discontinue Fabrazyme immediately and initiate appropriate medical treatment as needed. Assess the risks and benefits of readministering Fabrazyme and monitor patients closely if readministering.
- Patients with advanced Fabry disease may have compromised cardiac function, which may predispose them to a higher risk of severe complications from IARs. Closely monitor patients with compromised cardiac function receiving Fabrazyme.
Common Adverse Reactions
Adverse reactions reported (≥20%) were upper respiratory tract infection, chills, pyrexia, headache, cough, paresthesia, fatigue, peripheral edema, dizziness, and rash.
Please see full Prescribing Information, including Boxed WARNING
IMPORTANT SAFETY INFORMATION
Show moreIMPORTANT SAFETY INFORMATION
|
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy. Initiate FABRAZYME in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g. anaphylaxis) occurs, discontinue FABRAZYME and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur [see Warnings and Precautions (5.1)]. |
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions Including Anaphylaxis
In clinical trials and post-marketing experience, approximately 1% of patients developed anaphylactic or severe hypersensitivity reactions, some life-threatening, during Fabrazyme infusion. Reactions have included localized angioedema (including swelling of the face, mouth, and throat), bronchospasm, hypotension, generalized urticaria, dysphagia, rash, dyspnea, flushing, chest discomfort, pruritus, and nasal congestion. Consider pretreating with antihistamines, antipyretics, and/or corticosteroids; however, reactions may still occur.
In Fabrazyme clinical trials, some patients developed IgE antibodies or skin test reactivity specific to Fabrazyme.
- Higher incidences of hypersensitivity reactions were observed in adult patients with persistent anti-Fabrazyme antibodies, and in those with high antibody titers compared with antibody negative adult patients.
- Consider testing for IgE antibodies in patients who experienced suspected hypersensitivity reactions and consider the risks and benefits of continued treatment in patients with anti-Fabrazyme IgE antibodies. Rechallenge of these patients should only occur under the direct supervision of qualified personnel, with appropriate medical support measures readily available.
Infusion-Associated Reactions
In Fabrazyme clinical trials, 59% of patients experienced infusion-associated reactions (IARs), some of which were severe. IARs are defined as those occurring on the same day as the infusion. The incidence of these reactions was higher in patients who were positive for anti-Fabrazyme antibodies than those negative for anti-Fabrazyme antibodies.
- Consider pretreatment with antipyretics, antihistamines, and/or corticosteroids to reduce the risk of IARs; however, they may still occur.
- If a mild or moderate IAR occurs, consider holding the infusion temporarily, decreasing the infusion rate, and/or reducing the Fabrazyme dosage. If a severe IAR occurs, discontinue Fabrazyme immediately and initiate appropriate medical treatment as needed. Assess the risks and benefits of readministering Fabrazyme and monitor patients closely if readministering.
- Patients with advanced Fabry disease may have compromised cardiac function, which may predispose them to a higher risk of severe complications from IARs. Closely monitor patients with compromised cardiac function receiving Fabrazyme.
Common Adverse Reactions
Adverse reactions reported (≥20%) were upper respiratory tract infection, chills, pyrexia, headache, cough, paresthesia, fatigue, peripheral edema, dizziness, and rash.
Please see full Prescribing Information, including Boxed WARNING
INDICATION AND USAGE
Fabrazyme® is indicated for the treatment of adult and pediatric patients 2 years of age and older with confirmed Fabry disease.
